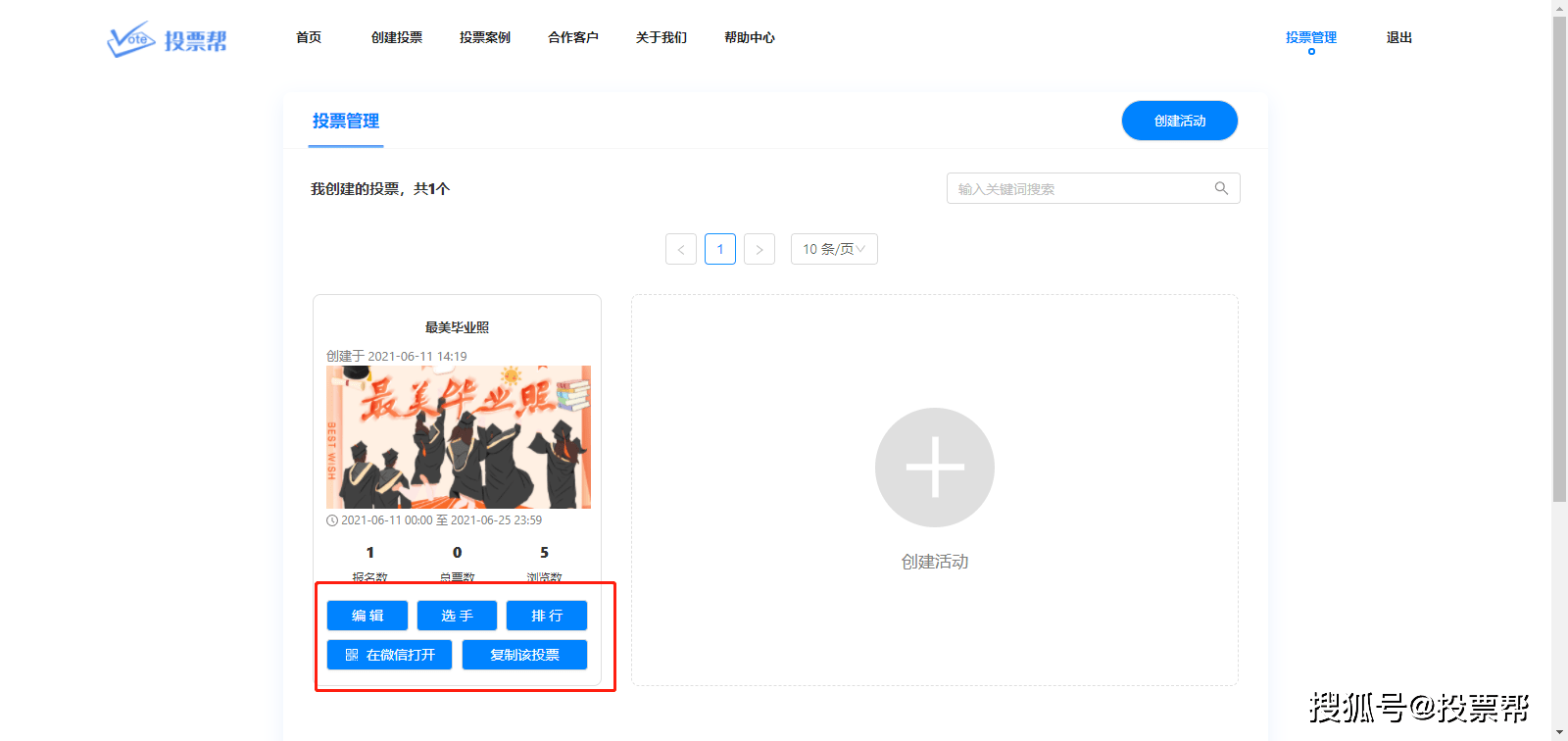
The World Health Organization (WHO) on June 1 approved the emergency use of a Covid-19 vaccine developed by Chinese pharmaceutical company Sinovac.
The WHO technical advisory group made the approval recommendation after reviewing the clinical data on the safety and effectiveness of the Sinovac vaccine as well as the company’s manufacturing operations, Reuters reported on 1/6.
In the statement, the WHO’s independent expert panel recommended that Sinovac be given to adults over 18 years of age, with a second dose initiated every 2-4 weeks. There is no age limit for this vaccine, as data shows it is likely to protect older adults. This is the second Chinese vaccine approved by the WHO for emergency use – after the Sinopharm vaccine approved in early May.  Covid-19 vaccine box manufactured by Sinovac pharmaceutical company. Photo: Anadolu Agency. A third Chinese vaccine, manufactured by CanSino Biologics, has submitted clinical trial data, but WHO has not scheduled a review. Sinovac said it had delivered more than 600 million doses of the vaccine at home and abroad as of the end of May, of which more than 430 million had been administered. In addition, China has deployed hundreds of millions of doses of its own vaccines – including Sinopharm and Sinovac – to many countries, especially in Latin America, Asia, and Africa. Many of these countries have difficulty finding a vaccine developed by the West.
Covid-19 vaccine box manufactured by Sinovac pharmaceutical company. Photo: Anadolu Agency. A third Chinese vaccine, manufactured by CanSino Biologics, has submitted clinical trial data, but WHO has not scheduled a review. Sinovac said it had delivered more than 600 million doses of the vaccine at home and abroad as of the end of May, of which more than 430 million had been administered. In addition, China has deployed hundreds of millions of doses of its own vaccines – including Sinopharm and Sinovac – to many countries, especially in Latin America, Asia, and Africa. Many of these countries have difficulty finding a vaccine developed by the West.


























































You must log in to post a comment.